bEnd.3 [BEND3]
Cat.No.: CSC-C9158W
Species: Mus musculus (Mouse)
Source: Brain
Morphology: endothelial
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
BEnd.3 cells are immortalized cells isolated from the cortex of mice with endothelial tumors, achieved through transfection with a retroviral vector carrying the polyoma middle T antigen. These cells can lead to hemangioma formation when injected into mice. Showing a typical epithelial-like morphology, BEnd.3 cells exhibit adherence growth characteristics, with tightly packed cells forming a monolayer network; at high-density cultures, they form a monolayer arranged in a whirlpool pattern.
Widely used to simulate the blood-brain barrier (BBB), BEnd.3 cells express various tight junction proteins, such as occludin and claudin-5, effectively blocking the permeation of radioactively labeled substances and demonstrating good responsiveness to disruptive stimuli. These cells also portray typical endothelial characteristics, including the expression of vascular endothelial growth factor (VEGF) receptors and the ability to uptake low-density lipoprotein (LDL). The cells are also employed in disease model research to study hemangiomas, the tumor microenvironment, and associated diseases.
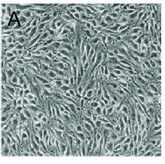 Fig. 1. Morphology of bEnd.3 cells (Sun ZY, Wang FJ, et al., 2019).
Fig. 1. Morphology of bEnd.3 cells (Sun ZY, Wang FJ, et al., 2019).
The Expression of Tight Junction Proteins of bEnd3 Cells is Regulated by Tp0751
Neurosyphilis progression is linked to T. pallidum crossing the BBB, yet the molecular mechanisms are unresolved. Tp0751, a T. pallidum adhesion protein, binds host receptors but its role in BBB disruption is underexplored. Lu's team explore the effect of the T. pallidum adhesion protein Tp0751 on the blood-brain barrier and cerebrovascular endothelial cells.
Immortalized bEnd3 cells were used in this study as they reflect cerebrovascular endothelial cell characteristics. Initially, they assessed Tp0751's impact on the tight junction proteins ZO-1, occludin, and claudin-5. QRT-PCR showed that Tp0751 influenced their transcription, while heat-inactivated Tp0751 and Tp0136 did not affect expression (Fig. 1A). Western blot analysis revealed changes in protein expression levels after bEnd3 cells were stimulated with Tp0751. Treatment with 5 μg/ml Tp0751 for 12 hours significantly reduced the expression of ZO-1, occludin, and claudin-5 (Fig. 1B). With increasing co-culture time, ZO-1 and occludin levels decreased, most notably at 18 and 24 hours, while claudin-5 increased at 18 hours (Fig. 1C). TEER tests showed a non-significant decrease in BBB model integrity (Fig. 2B), and TEM showed no structural damage to tight junctions (Fig. 2C–2D).
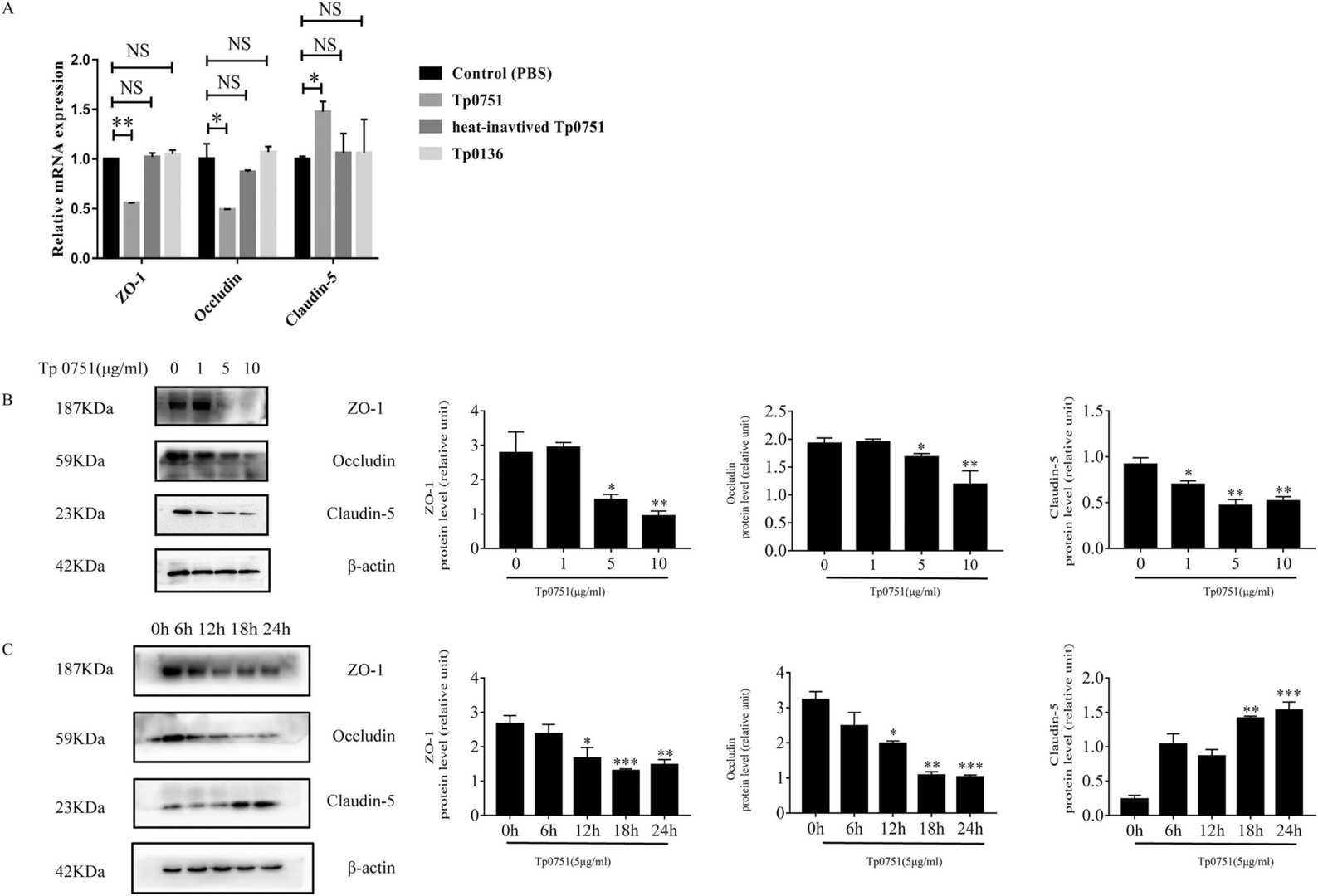 Fig. 1. Treponema pallidum recombinant protein Tp0751 affects the expression of tight junction proteins in bEnd3 cells (Lu S, Wang J, et al., 2022).
Fig. 1. Treponema pallidum recombinant protein Tp0751 affects the expression of tight junction proteins in bEnd3 cells (Lu S, Wang J, et al., 2022).
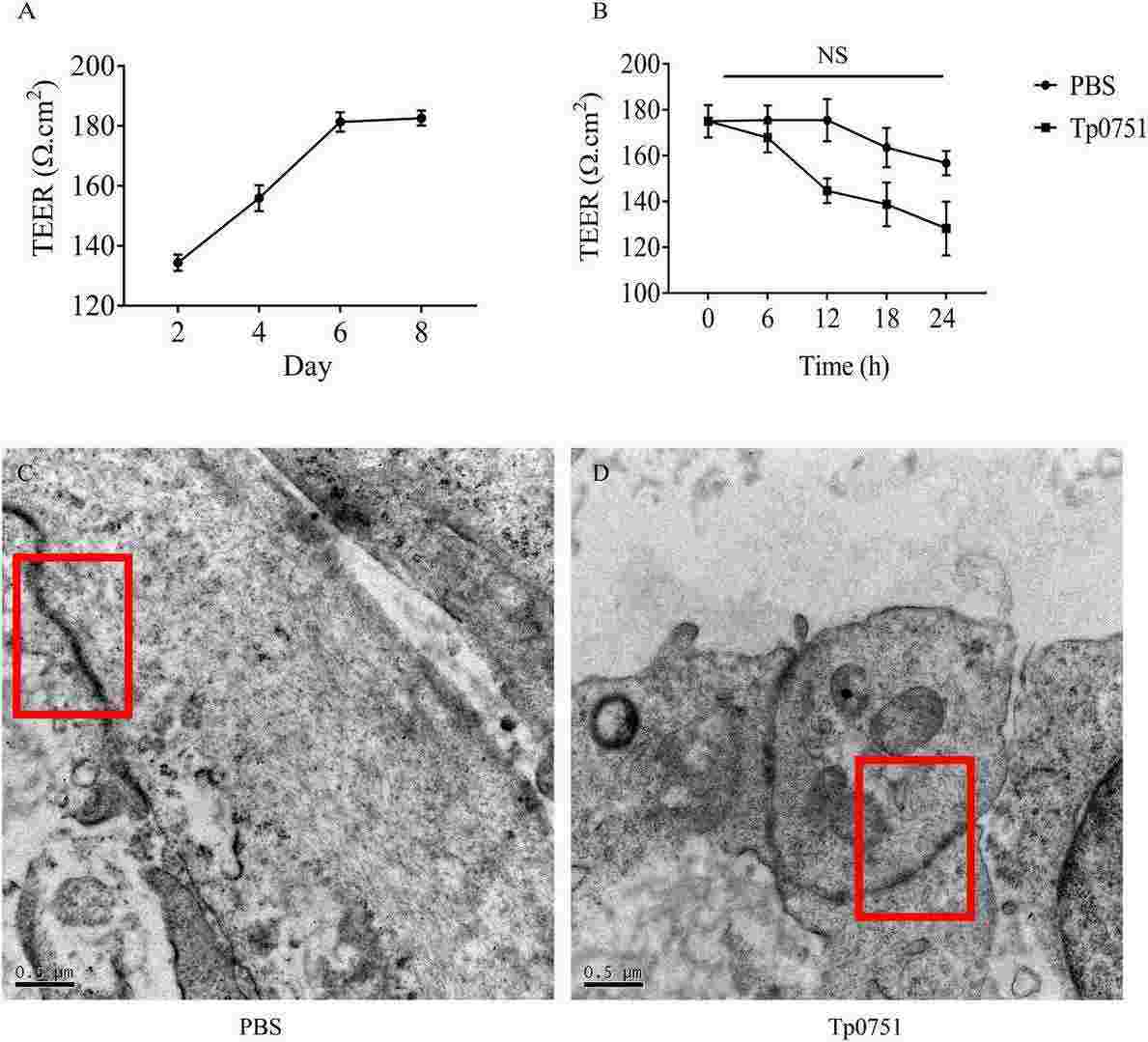 Fig. 2. Tp0751 had no significant effect on the TEER of bEnd3 cells, and it did not significantly destroy the tight junction structure between bEnd3 cells (Lu S, Wang J, et al., 2022).
Fig. 2. Tp0751 had no significant effect on the TEER of bEnd3 cells, and it did not significantly destroy the tight junction structure between bEnd3 cells (Lu S, Wang J, et al., 2022).
IH-Induced Exosomes Promote Pyroptosis and Inflammation in HT22 Cells
OSA, characterized by IH, leads to CI possibly due to BBB disruption and pyroptosis. Exosomes, rich in miRNAs, play a role in cell signaling potentially linked to OSA-CI. Chen's team aims to investigate whether miR-20a-5p in exosomes derived from bEnd3 cells with IH mediates intercellular crosstalk and induces CI through hippocampal neuronal cell pyroptosis.
Increased pyroptosis has been noted in OSA patients, but it's unclear if IH-induced exosomes contribute to this. IH-EXOS was co-incubated with HT22 cells to examine pyroptosis. Compared to control and NC-EXOS, IH-EXOS significantly raised GSDMD, Caspase-1 protein, and mRNA levels in HT22 cells. Additionally, inflammatory cytokines IL-1β, IL-6, IL-18, and TNF-α were elevated, likely due to pyroptosis-induced inflammation (Fig. 3A–C). To confirm exosome involvement, GW4869, an exosome secretion inhibitor, was added to bEnd3 cell cultures. This reduced IH-EXOS's effects on HT22 cells, showing decreased GSDMD, Caspase-1, and inflammatory factors (Fig. 3A–C). These findings indicate IH-EXOS can induce HT22 cell pyroptosis and activate inflammation, possibly causing neuronal cell death.
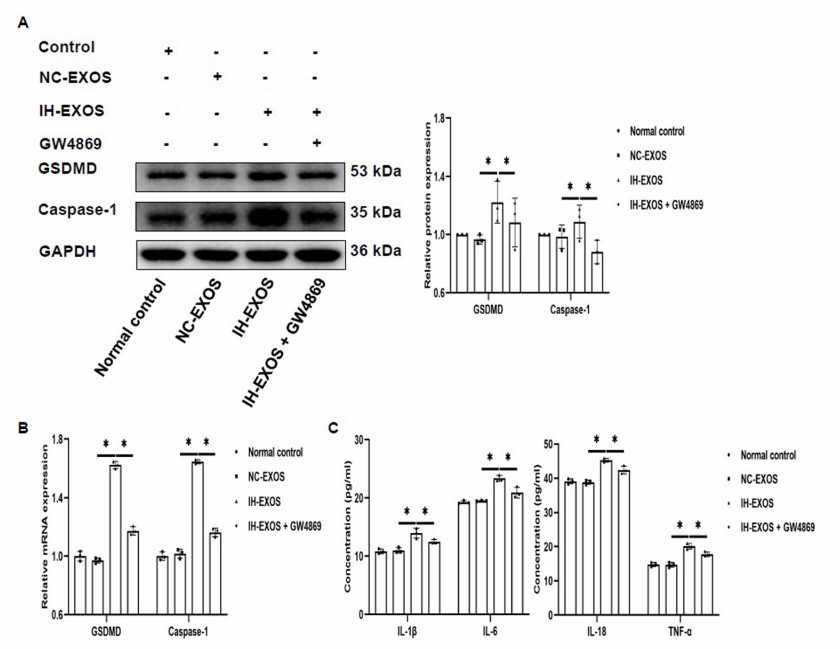 Fig. 3. IH-induced exosomes promote pyroptosis and inflammation in HT22 cells (Chen ZF, Shang YL, et al., 2024).
Fig. 3. IH-induced exosomes promote pyroptosis and inflammation in HT22 cells (Chen ZF, Shang YL, et al., 2024).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells